Esophageal adenocarcinoma (EAC), one of two major forms of esophageal cancer, is the sixth most deadly cancer worldwide for which no effective targeted therapy exists. Patients need to rely on chemotherapy as a standard-of-care, which is started ahead of surgical interventions as a so-called “neoadjuvant chemotherapy” (NACT) in the hope to shrink or control tumors. However, most patients become resistant to certain NACTs, leading to poor outcomes.
Given the utter lack of therapeutic alternatives, responders and non-responders alike, continue to receive one of the available chemotherapies without knowing whether it will work. Even in responders, the chemotherapy of choice may not completely stop their tumors from progressing and metastasizing, and it can have toxic side effects on the body. The availability of a personalized, patient-specific precision oncology model that can accurately predict a patient’s response to different NACTs in a timely manner is a critical unmet need.
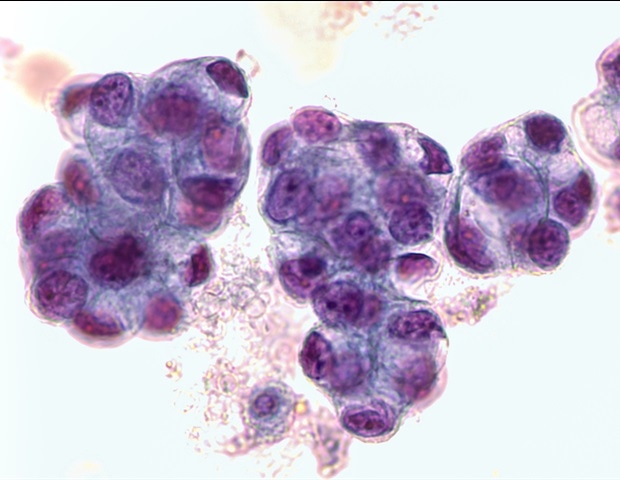
Researchers had grown so-called “organoids” from biopsied EAC cells, which are 3D esophageal mini-organs formed with tissue-specific stem cells that exhibit critical features of the esophageal epithelial lining. However, these lack important components of a patient’s specific tumor microenvironment (TME), such as the stromal fibroblasts and collagen fibers, and thus, they do not show the same responses to NACT as actual tumors.
Read the full story here
.