By Godfred Aaneamenga Polkuu
Bolgatanga, May 30, GNA –Mr Abel Ndego, Acting Head of the Food and Drugs Authority (FDA) in the Upper East Region, has cautioned supermarkets and shop owners against the sale of dented products in the Region.
He said the FDA would not bend the rules or compromise on public health and safety, but would continue to insist that products in supermarkets, shops and malls across the Region were in good condition for public consumption.
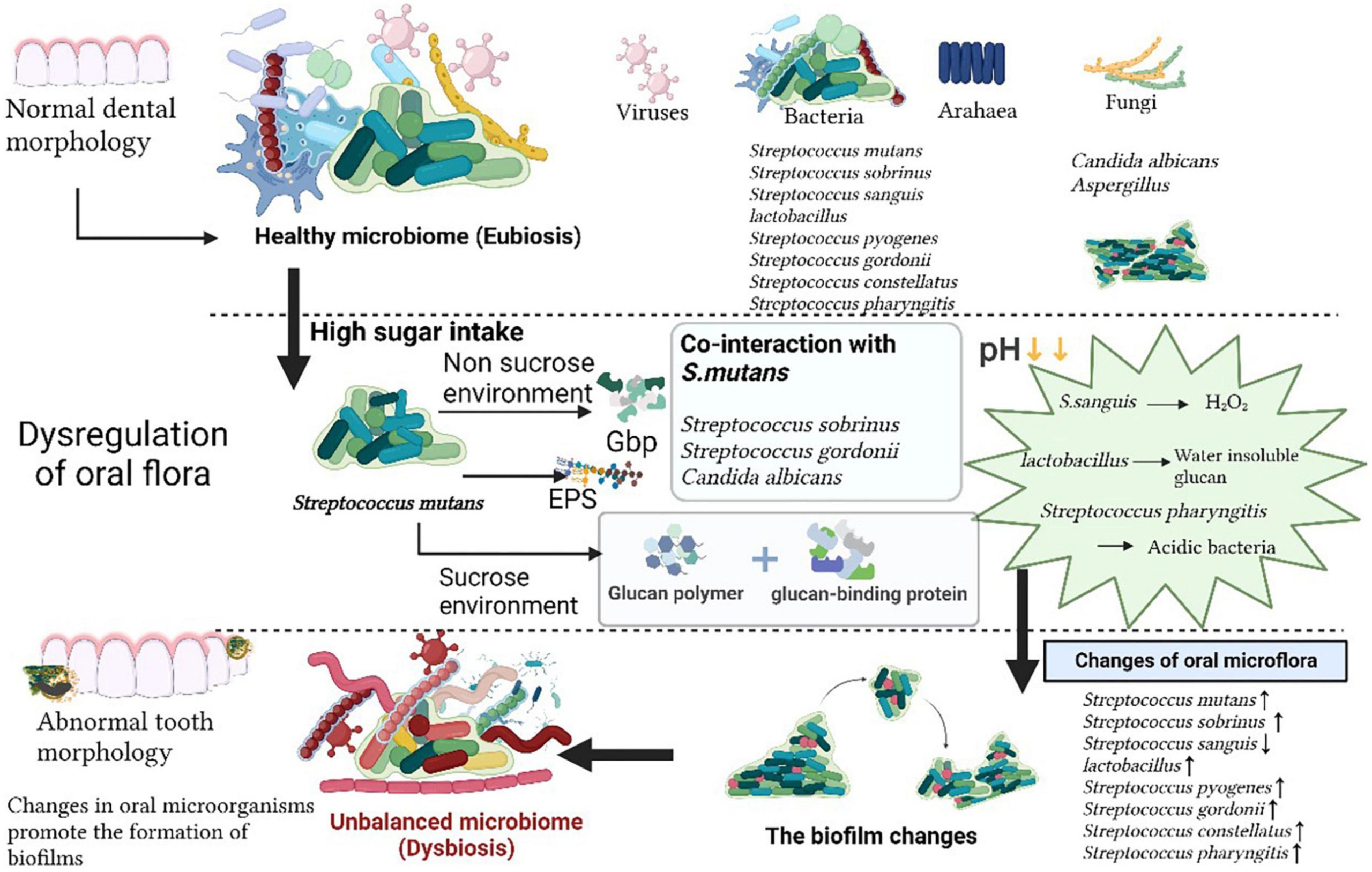
A “dented product” refers to an item, often in retail, that has sustained some damage, particularly a small indentation or depression, resulting from shipping or handling. This damage is usually cosmetic and doesn’t affect the product’s functionality, but it often leads to a reduced price.
“We will not permit you to sell any form of dented products,” Mr Ndego said.
Mr Ndego’s caution was in response to questions by shop operators during a training programme on good storage and distribution practices in the Upper East Region.
The programme, organised by the FDA, afforded officials the opportunity to sensitize operators, drawn from across the 15 Municipalities and Districts in the Region on part seven of the Public Health Act 2012; Act 851, and good storage and distribution practices.
Mr Ndego explained that manufacturers of products, especially canned products galvanized the inner metals of their containers to prevent direct contact with the products, which could pose health risks to members of the public if the continuity of the galvanized seal was broken.
Using canned tomato paste for example, he said: “When there is a dent, it breaks the continuity of the seal and creates direct exposure or contact between the canned tomatoes and the metal, with dangerous chemicals introduced into the tomato paste, and that is harmful for human consumption.
“This principle holds for all canned products; whether it is condensed or liquid milk, canned fish and anything canned,” he said.
Asked by an operator if they could keep expired products and show to companies and suppliers as evidence and later dispose them, Mr Ndego said: “Nobody has the right to dispose of any regulated products without the strict and express supervision of the FDA.”
He noted that the FDA could not risk leaving unwholesome, regulated products with shop operators, and said the Authority issued a detention notice; a document that highlighted details of all the products to them, and the reason for detention.
“We even give you the original copy, and we keep the duplicate. That is evidence that FDA has taken this quantum of products from you, with reasons, so that when the company or suppliers come, you show the detention notice to them as evidence that you didn’t sell the products, but FDA took them,” he said.
Ms. Joyce Agana, Principal Regulatory Officer of the FDA, who took participants through good storage and distribution practices, observed that some shop operators combined products meant to be frozen and those to be refrigerated in one refrigerator under the same temperature, instead of separating them for proper storage.
She said some products for distribution, especially locally made yoghurt products which should be stored under cold chain, were not, and as a result, the products fermented, and admonished the operators to ensure such products were transported in coolers with ice cubes.
Ms. Agana further advised participants against the unhygienic conditions under which some shops operated, and urged operators to strictly abide by proper hygiene practices for public safety.
Some participants who spoke to the Ghana News Agency after the programme, admitted they were knowledge deficient on some laws and regulations of the FDA and its prescribed safety precautions.
They commended the management of the Authority for the training and called for more training programmes to serve as a reminder to them to play their roles as stakeholders to ensure public safety.
GNA
Edited by Fatima Anafu-Astanga/Benjamin Mensah
Provided by SyndiGate Media Inc. (
Syndigate.info
).