A Breakthrough in Brain Organoid Research
An interdisciplinary team working on balls of human neurons called organoids aimed to expand their research and tackle significant new questions. The solution was all around them.
For almost a decade, the Stanford Brain Organogenesis Program has pioneered a revolutionary approach to studying the brain: instead of examining intact brain tissues in humans and other animals, they grow three-dimensional brain-like tissues in the lab from stem cells, creating models known as human neural organoids and assembloids.
Starting in 2018 as a Big Ideas in Neuroscience project of Stanford’s Wu Tsai Neurosciences Institute, the program has brought together neuroscientists, chemists, engineers, and others to explore various aspects such as the neural circuits involved in pain, genes that drive neurodevelopmental disorders, and new ways to study brain circuits.
Despite these advancements, one major challenge persisted: scale. If researchers could produce thousands of organoids at once with uniform size and shape, they could gain deeper insights into brain development and developmental disorders, and more efficiently test new drugs and gene therapies.
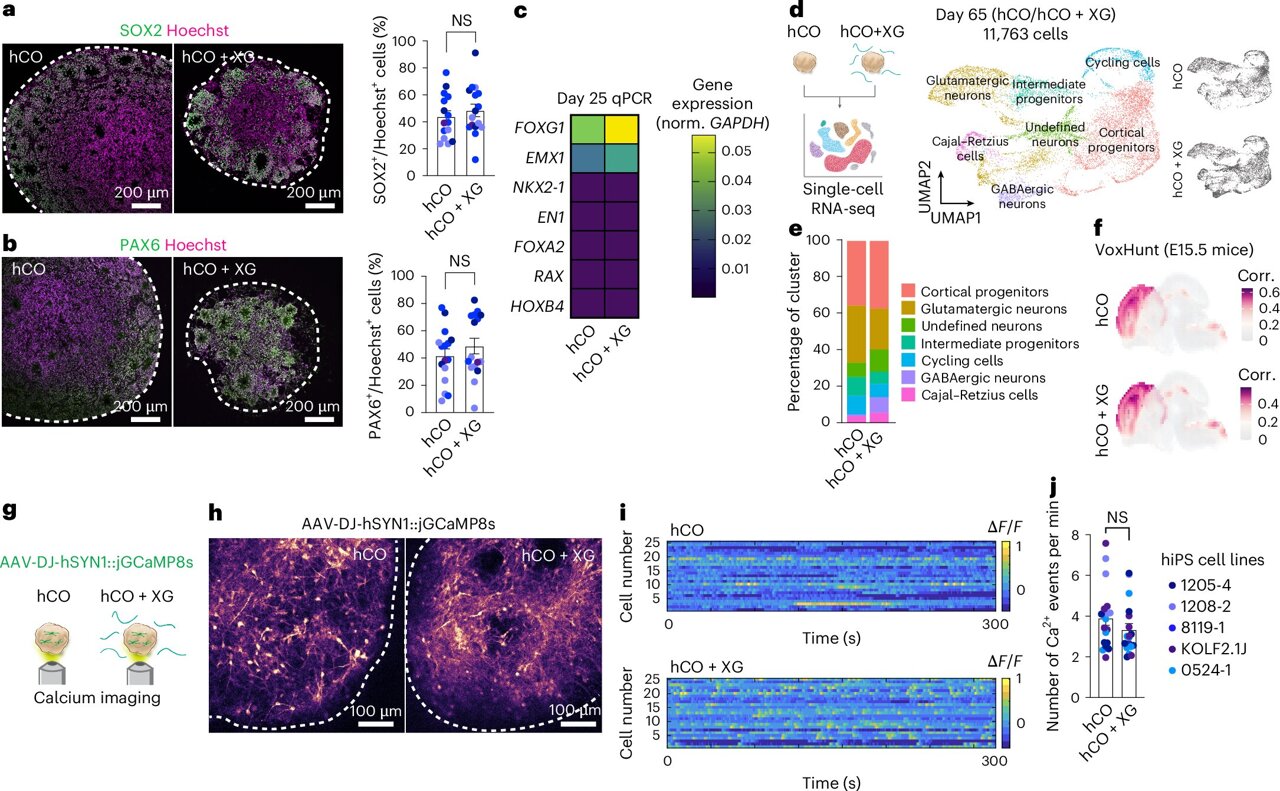
The problem was that neural organoids tend to stick to each other, making it difficult to grow large batches with consistent size and shape. However, a team of neuroscientists and engineers led by Sergiu Pasca, the Kenneth T. Norris, Jr. Professor of Psychiatry and Behavioral Sciences in the School of Medicine, and Sarah Heilshorn, the Rickey/Nielsen Professor in the School of Engineering, found a simple solution. Their study published June 27 in Nature Biomedical Engineering revealed that xanthan gum, a common food additive, effectively prevented organoids from sticking together.
“We can easily make 10,000 of them now,” said Pasca, the Bonnie Uytengsu and Family Director of the Stanford Brain Organogenesis Program. In line with the program’s commitment to making their techniques widely available, they have already shared their approach so others can benefit. “This, as with all of our methods, is open and freely accessible. There are already numerous labs that have implemented this technique.”
The Evolution of Organoid Research
Things weren’t always so easy. About a dozen years ago, Pasca had just developed a method for transforming stem cells into the three-dimensional tissues now known as regionalized neural organoids, and he could only manage to make a few of these early cultures. “In the early days, I had eight or nine of them, and I named each of them after mythological creatures,” Pasca recalled.
But Pasca wanted to understand what happens during brain development that leads to disorders like autism or Timothy syndrome, and to explore ideas such as screening drugs for potential side effects on brain development. To do that, he said, “we needed to produce thousands of organoids, and they should all be the same.” He also realized he would need to involve a wide range of researchers. “I thought, ‘This is an emerging field and there are a lot of problems we’re going to face, and the way we’re going to face them and solve them is by implementing innovative technologies.'”
To move forward, Pasca partnered with Karl Deisseroth, a bioengineer and neuroscientist, and brought together an interdisciplinary team to launch the Stanford Brain Organogenesis Program with support from Wu Tsai Neuro’s Big Ideas in Neuroscience grant.
Overcoming the Stickiness Problem
The stickiness problem emerged soon after. Organoids were fusing together, resulting in smaller numbers of organoids of different shapes and sizes. “People in the lab would constantly say, ‘I made a hundred organoids, but I ended up with 20,'” Pasca said.
While this presented both a blessing and a curse, the team still needed to create large numbers of organoids to gather precise data on brain development, screen drugs for growth defects, or carry out other projects at scale. One possibility would be to grow each organoid in a separate dish, but doing so is often inefficient. Instead, the lab needed something to keep organoids apart while growing them in batches.
Pasca worked with Heilshorn, a materials engineer, to try out various options. The team tested 23 different materials, selecting those that were biocompatible, economical, and simple to use so that their methods could be adopted easily by other scientists.
Demonstrating the Potential of the Technique
To demonstrate the technique’s potential, the team used it to address a real-world issue: doctors often hesitate to prescribe potentially beneficial drugs to pregnant people and babies due to concerns about their impact on developing brains. Co-lead author Genta Narazaki, a visiting researcher in Pasca’s lab, grew 2,400 organoids in batches and added one of 298 FDA-approved drugs to each batch to see if any caused growth defects. Working closely with co-lead author Yuki Miura, Narazaki showed that several drugs, including one used to treat breast cancer, stunted the growth of the organoids, suggesting they could be harmful to brain development.
“This experiment shows that researchers could uncover potential side effects—and do so very efficiently,” Pasca said. “One single experimenter produced thousands of cortical organoids on their own and tested almost 300 drugs.”
Pasca and his colleagues are now hoping to use their technique to make progress on a number of neuropsychiatric disorders, such as autism, epilepsy, and schizophrenia. “Addressing those diseases is really important, but unless you scale up, there’s no way to make a dent,” Pasca said. “That’s the goal right now.”